|
ACID RAIN |
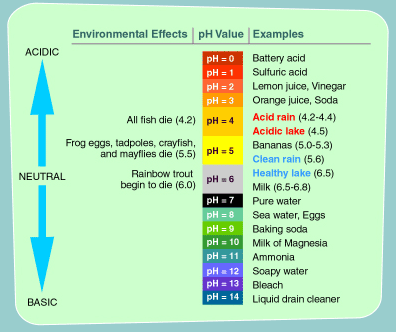
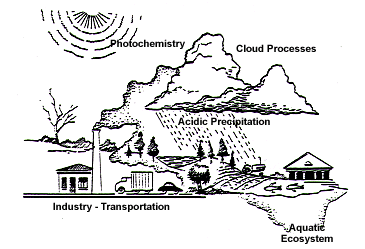 Acid Rain is a little talked about atmospheric problem directly linked to the burning of most hydrocarbons in industrial and vehicular applications.
> first identified by Dr Harvey Uof T
> when hydrocarbons undergo combustion S and N compounds
are generated that travel throughout the atmosphere to distant locations
and fall in precipitation as acid rain
S + H2O => H2SO4 sulphuric acid
(unbalanced)
>>
http://www.on.ec.gc.ca/community/classroom/c4-acid-rain-e.html
|