|
|
||||||||||||||||||
|
Our planet would better be
called Water Earth after all is 70% covered in water
at an average depth of 3km although most abundant life occurs in the
shallows. Characteristics and important qualities in regard to life on Earth
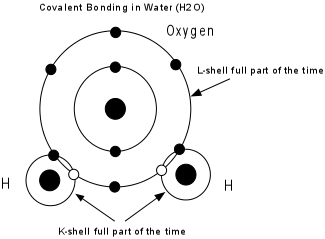
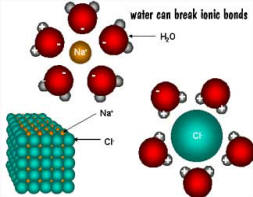
1) water is a universal solvent , can
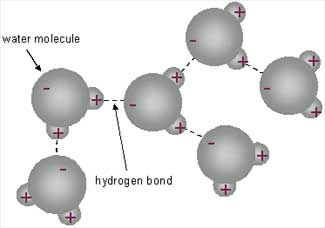
form weak bonds with with other polar molecules and can break (ionize)
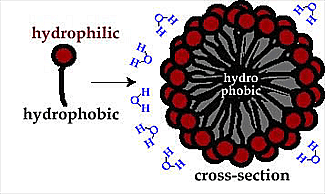
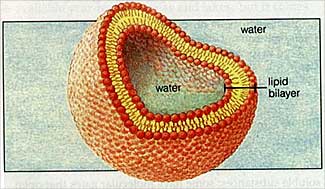
2) Water organizes molecules leading to such things as oil droplets and cell membranes ...... in a tendency to minimize system energy levels
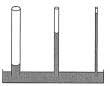
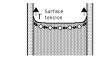
3) surface tension
allows insects to skate across the surface of water , bird feathers can
repel water
4) Energy is required for change
of state
7) Acid formation - water is
an
acid and a base and is suitable environment for acid formation
|